The CCHFVaccine project, has been built on the success of the CCH Fever project (FP7), which had focused on improving the knowledge of CCHFV, in general.
The specific aim of the CCHFVaccine project is to develop and deliver a vaccine, which can significantly increase our capacity to control the situation of Crimean Congo Haemorrhagic fever (CCHF) disease on a global basis.
The vaccine candidates developed within this project will not only be developed for human use but also for domestic animals in endemic and non-endemic areas.
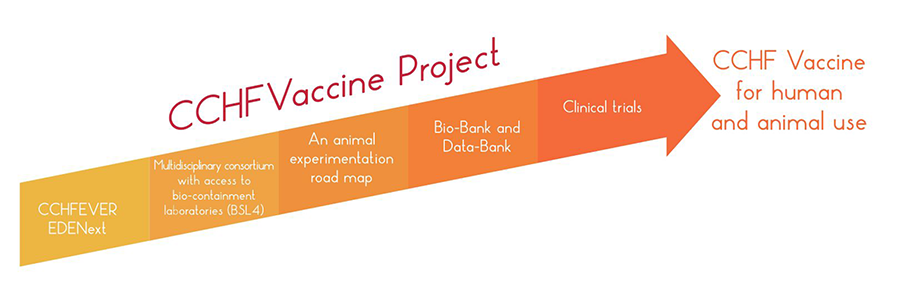
To achieve this overall aim, an intensive work plan (based on the previous CCH Fever and EDENext project results) will be put in place with the following specific objectives:
|
|
Objective 1: to produce already available and promising vaccine candidates and also further establish new vaccine candidates for CCHFV for animal experimentation (WP2). |
|
|
Objective 2: to bring several unique animal models into front line vaccine research and to implement a roadmap for animal model evaluation (via WP3). |
|
|
Objective 3: to validate and bring the most promising vaccine candidates to clinical trials (via WP3). |
|
|
Objective 4: to ensure that an immune mediated protection is adequately understood and that the candidate vaccine(s) can elicit an appropriate and protective immune response (via WP4). |
|
|
Objective 5: to establish a clinical trial road map and perform clinical trials at Phase I for the most promising platform. In addition ensure a strategy for the effective deployment, utilisation in resource-poor settings, field-deployment logistics and an evaluation of the predicted cost and affordability of the final vaccine products (via WP5). |
|
|
Objective 6: to widely publicise the project and its results to public health bodies, NGOs,outbreak management teams and develop an exploitation plan (via WP6). |


